How To Guide for E.O. 12866 Meetings
Meetings on a Regulatory Action under Review with the Office of Information and Regulatory Affairs (E.O. 12866 meetings)
OIRA is an office located within the Office of Management and Budget (OMB) in the Executive Office of the President. OMB supports the President in overseeing the implementation of policies across the Federal government.
Within OMB, OIRA manages the process of government-wide review of Federal regulatory actions. OIRA reviews a subset of regulatory actions, according to a series of Executive Orders, including Executive Order 12866, Executive Order 13563, and Executive Order 14094.
OIRA may review regulatory actions that are likely to have large impacts on the economy (e.g., annual effect on the economy of $200 million or more) or to ensure consistency in regulatory approaches across Federal agencies, among other circumstances. OIRA works collaboratively with Federal agencies through this review to ensure that regulatory actions are effective and durable.
Yes. When OIRA is reviewing a draft regulatory action, any member of the public can request a meeting with OIRA officials on that action. These meetings are sometimes called "12866 meetings," named after the Executive Order that structures OIRA's centralized regulatory review.
In many cases, OIRA will review a draft regulatory action twice: once as a proposal (typically called a "proposed rule"), and again when the agency is finalizing the action (typically called a "final rule"). Members of the public can request 12866 meetings with OIRA in both cases.
Note, however, that a 12866 meeting with OIRA is not a substitute for submitting written comments to the agency that is proposing the regulatory action. Members of the public should follow the instructions set forth in the agency's rulemaking document to understand how, and by when, they should submit a comment.
In addition, not all regulatory actions are reviewed by OIRA; if a regulatory action is not reviewed by OIRA, then 12866 meetings with OIRA on that action will not be available.
12866 meetings can be scheduled in a number of ways, as detailed below: Option 1 requires knowledge of the regulatory action's Regulatory Identification Number (RIN); Options 2 and 3 do not require such information. A RIN consists of a 4-digit agency code plus a 4-character alphanumeric code designed to identify an individual regulatory action (e.g., 1234-XX56). Agencies use RINs to identify individual regulatory actions in the semi-annual Unified Agenda of Regulatory and Deregulatory Actions, as well as in notices published in the Federal Register.
Each of the options below takes the meeting requester to the meeting scheduling form. After OIRA receives your scheduling request, you will be sent an email from our scheduler and must follow the instructions in that email to confirm your meeting. If you fail to confirm your meeting within 2 working days (when feasible), your meeting may be canceled. If you confirm, but do not attend, this will also be noted in the public record. While OIRA makes every effort to accommodate meeting requests, OIRA may finish review of a regulatory action before a scheduled meeting. In that case, OIRA may need to cancel the meeting. OIRA will make every effort to notify requesters as soon as possible if a meeting needs to be canceled.
If the meeting requester already knows the RIN for the regulatory action, the requester can click here to enter the RIN and then be taken to a meeting scheduling page for that regulatory action. Requesters can then follow the instructions on that page to verify their email address and complete their meeting request.
Alternatively, the requester can enter the RIN directly on the meeting request form, which can be found on OIRA's main website at www.reginfo.gov, by clicking on the "Regulatory Review" tab and then selecting "EO 12866 Meetings" (as circled in black below).
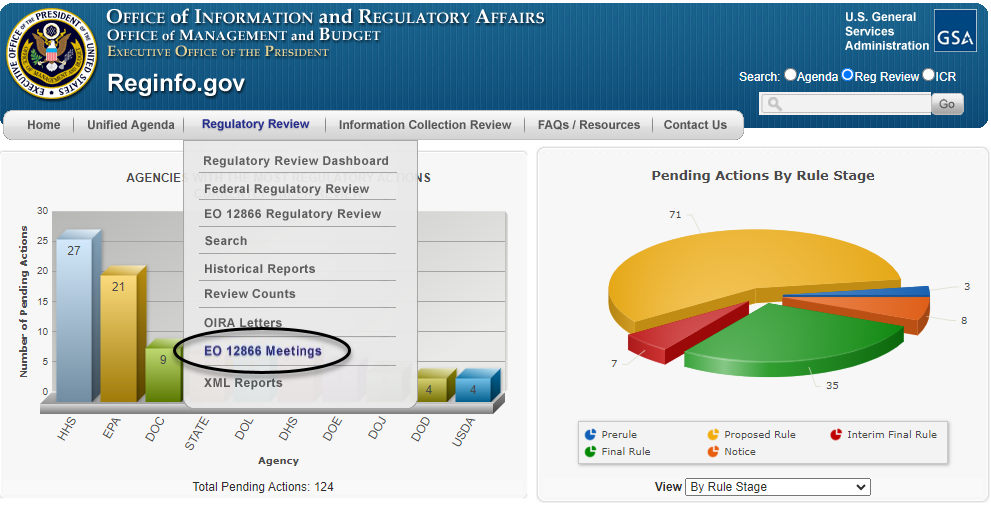
Clicking on "EO 12866 Meetings" then takes the requester to the search page shown below, where there is a button labeled "Schedule" near the top of the page (as circled below) for requesting a meeting.
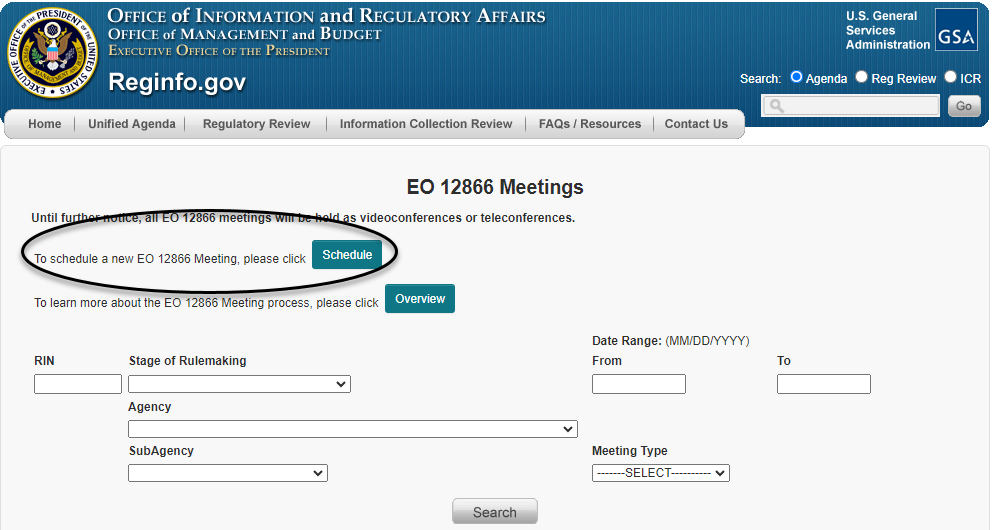
Clicking on that "Schedule" button then takes the meeting requester to a page (as shown below) asking the requester to identify the RIN of the regulatory action. Once the RIN is entered, the requester will be taken to a meeting scheduling form.
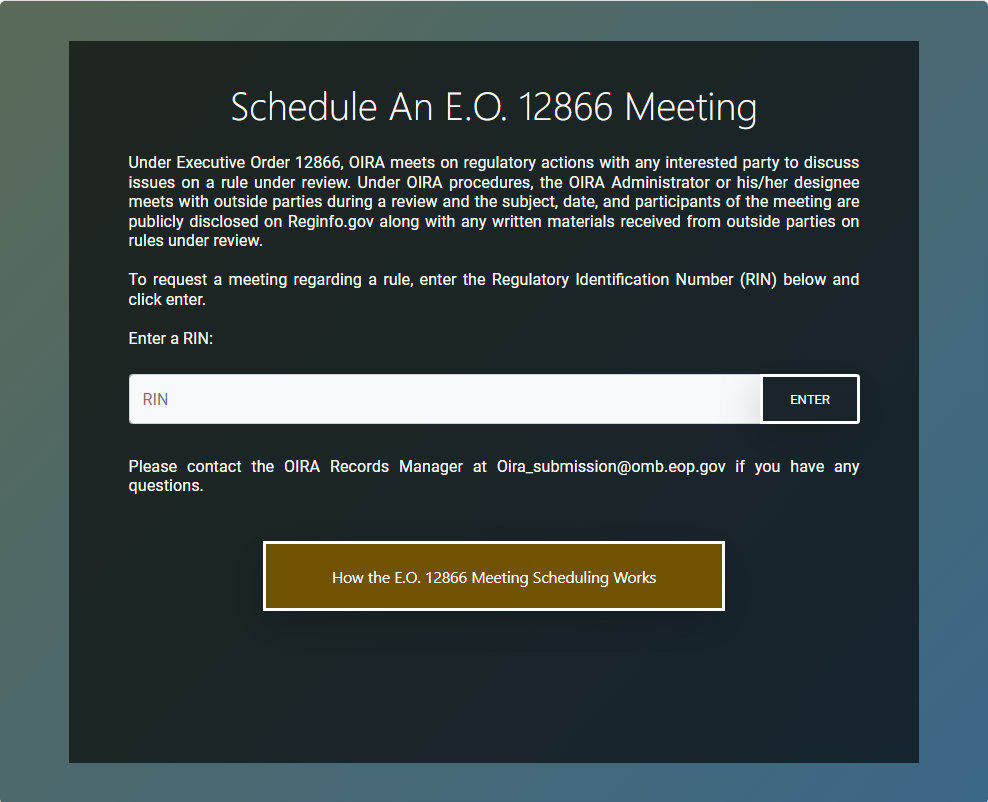
If a meeting requester does not know the regulatory action's RIN, they have at least two other options, described in more detail below, involving either (1) browsing the list of all pending reviews or (2) searching for the regulatory action's individual entry.
This option allows the meeting requester to browse a list of pending reviews to locate the regulatory action of interest and then select the option to "Request EO meeting" from that entry. More specifically, meeting requesters can click here to see a list of all pending reviews, or click on any part of the infographic titled "Pending Actions by Rule Stage" (as circled below) to be taken to the same page.
Either route brings up a list of pending regulatory actions currently under review, an illustrative example of which is below. The list can be sorted by agency or by rule stage to help the requester locate a regulatory action. Once the regulatory action has been identified, the requester can click the "Request EO Meeting" link to the right of the entry (and circled below) to be taken to the meeting scheduling form.
Another option for scheduling a meeting is by searching for the individual entry for the regulatory action of interest. To do so, the meeting requester can first choose the "Search" option under the "Regulatory Review" tab (as circled below).
There, the requester can search regulatory actions that are pending under review by choosing the button on the right side of the screen labeled "Pending Review" under "Review Status" (as circled below).
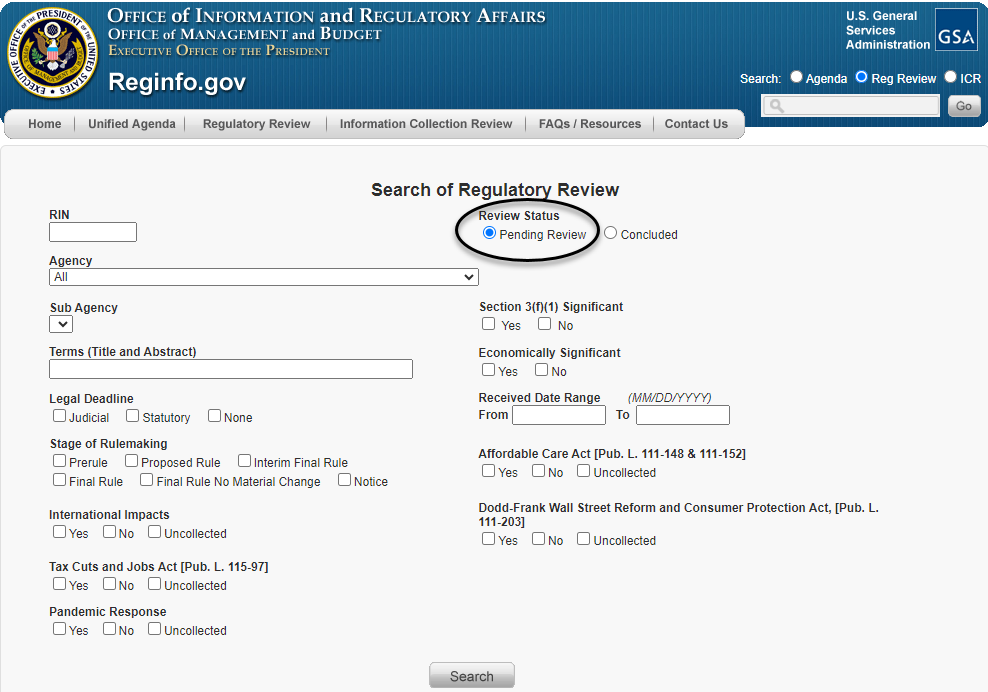
The requester can then narrow the search results through a number of fields, as shown above, including the agency or sub-agency; terms within the title or abstract of the rule; or stage of the rulemaking process, among other fields. Once that action has been identified, the requester can click on the "Pending Review" hyperlink to the right (as circled below) to be taken to the regulatory action's individual entry.
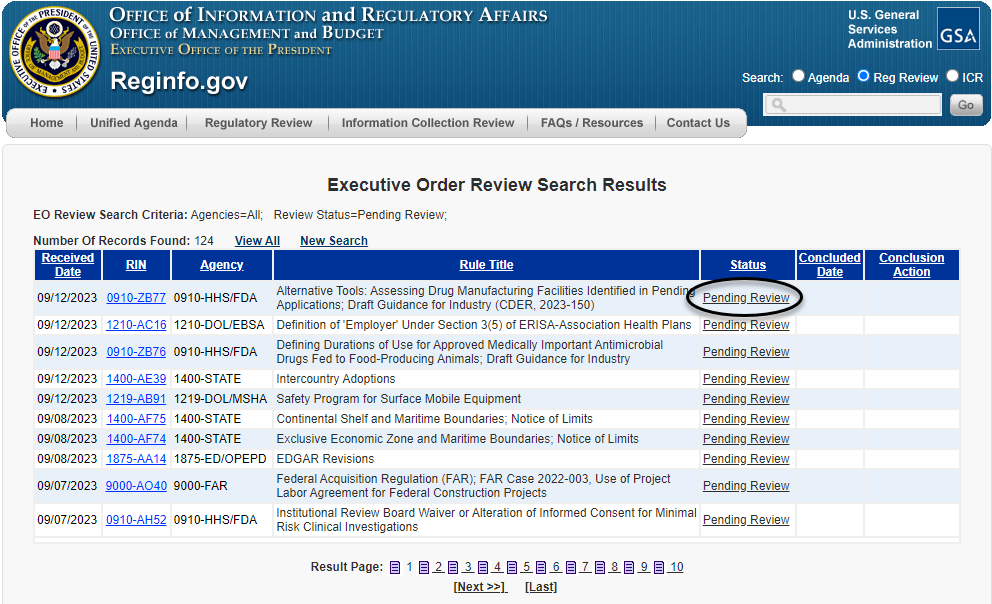
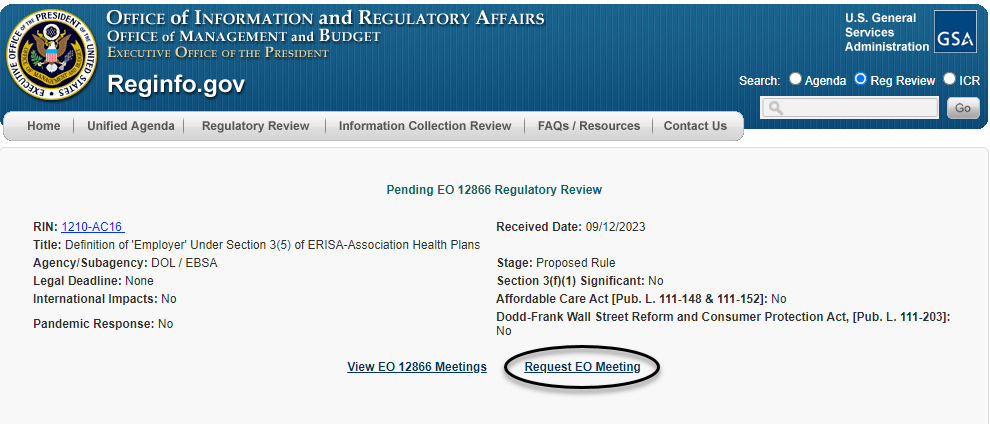
Once taken to the regulatory action's individual entry, a requester can then click on the "Request EO Meeting" hyperlink, as circled below, to be taken directly to the meeting schedule form.
OIRA staff attend these meetings, and may also invite representatives from the Federal agency that is taking the regulatory action to join. For example, if you request a 12866 meeting with OIRA on a regulatory action proposed by the Department of Labor, OIRA will invite staff from the Department of Labor to participate. These meetings are scheduled for 30 minutes. As noted above, a 12866 meeting is not a substitute for submitting written comments to the agency taking the regulatory action.
Please note that to encourage transparency about OIRA's review of regulatory actions, OIRA publishes information about who requests and attends meetings such as attendees' names and organizational affiliations, if any, as well as materials shared by members of the public at those meetings. You can view this information here.
Requesters may add/modify attendees and upload any relevant documents prior to their scheduled meeting. The email that requesters receive from OIRA once they have submitted their initial request contains information on how to add or modify attendees and upload supporting documents or materials.
Requesters cannot add attendees or materials on the day of the meeting, but can upload additional documents or names for up to 24 hours after the meeting concludes. OIRA will also update website information on attendees and materials provided at the meeting as necessary.
OIRA currently conducts 12866 meetings with the public as telephonic or virtual meetings (through WebEx or Zoom). At the start of the meeting, OIRA staff will introduce members of the Federal government who are on the call. OIRA will then turn over the floor to the meeting requester(s) to present their views.
OIRA does not provide a copy of the regulatory action currently under review. In addition, members of the government will be in "listening mode" and cannot provide details about or answer questions regarding the substance of the regulatory action. Members of the government may, however, ask clarifying questions in response to information provided by the meeting requester.
Your perspectives in these meetings are most useful to the Federal government if they are evidence-based or reflect your own lived experiences with government policies. For instance, you might share how a regulatory action could impact your family's life, or the way that your small business works.
You may also point out issues of importance, emphasize the value of alternative approaches and/or substitute language, and help decision-makers appreciate the strengths of other solutions than those proposed. If you are personally affected by an issue in a regulatory action or work with people who are affected, you may also use this meeting as an opportunity to highlight your first-hand experiences.
No. 12866 meetings with OIRA are not a substitute for submitting written comments to the action agency (meaning the agency taking the regulatory action) under its applicable regulatory procedures. Views and information shared only through a 12866 meeting are not considered properly submitted comments to the action agency during the notice and comment period, and need not be considered by the action agency when reviewing and responding to public comments. If a commenter wants to ensure an issue is considered by the action agency and is part of the action agency's administrative record, the issue must be raised with the action agency during the action agency's notice-and-comment period under its applicable regulatory procedures.
In general, we suggest the following considerations as you are planning for your meeting with OIRA:
- Consider offering views on targeted issues. Regulatory actions often touch on many issues. In your meeting, you do not need to share views on every issue. We encourage you to select those issue(s) of greatest concern to you and/or affect you the most and/or that you understand the best.
- Consider offering specific examples and alternatives. If possible, include specific examples of how you or your organization would be impacted negatively or positively and, if applicable, provide references to any reports, articles, data, or other source material supporting your position. If you disagree with a proposed agency regulatory action, consider suggesting an alternative (including not regulating at all) and include an explanation and/or analysis of how the alternative might meet the same objective or be more effective.
- Consider offering your reasoning. This meeting is not a voting process to determine which proposals have the most support from meeting requesters, but a chance to hear relevant information from members of the public. The government is attempting to make the best policy, so when discussing your views (including any materials you provide to the government) it is important to explain the reasoning behind your position. Views on the effects of rules-including quantitative or qualitative data about effects on potential economic, environmental, public health and safety, or other considerations; distributive impacts; equity; or other costs and benefits-can be particularly helpful to OIRA's review. We note that agencies often ask specific questions or raise issues in regulatory proposals on subjects where they are actively looking for more information, so we encourage you to consider these issues.
 An official website of the United States government
An official website of the United States government